Medical imaging encodes information of underlying tissues and can provide a comprehensive view of the entire body repeatedly throughout the course of disease. Therefore, medical imaging has been the foundation of disease detection and follow-up in clinical practice for decades. However, to date, observer-dependent evaluation of medical imaging has been a constraint for developing imaging biomarkers towards precision medicine. The application of advanced computational analysis to imaging data opens up a new paradigm in the radiology field.
Digital imaging data can be computed by the application of mathematical algorithms allowing extraction of a substantial amount of information about the tissue intensity, shape and texture, providing an excellent tool for improving imaging data interpretation and biomarker development. However, the considerable excitement resulting from the first-ever studies in radiomics has been followed by an awareness of flaws in radiomics reproducibility, particularly when gathering scans in retrospective or multi-centre studies, due to diversity in image acquisition and processing protocols.
Different approaches have been considered in order to overcome this issue, including non-reproducible feature refinement or incorporation of image-acquisition parameters as a confounding variable into the models. In our study, we aimed to bring light to the veritable value of CT-derived hand-crafted radiomics robustness and the sources of radiomics variability. Not only that, we propose a new pipeline for CT-image standardization based on image post-processing and hand-crafted radiomics normalization by the application of batch-effect correction tools. The described methods expand the potential of CT radiomics implementation in retrospective and prospective multicentre large-scale studies. These may lead to meaningful generalizable CT radiomics-based assays for supporting medical decisions in clinical practice.
Key points
- The voxel size (accounting for the pixel size and slice spacing), slice thickness, and convolution kernel are relevant sources of CT-radiomics variability.
- Voxel size resampling increased the mean percentage of robust CT-radiomics features from 59.50 to 89.25% when comparing CT scans acquired with different pixel sizes and from 71.62 to 82.58% when the scans were acquired with different slice spacings.
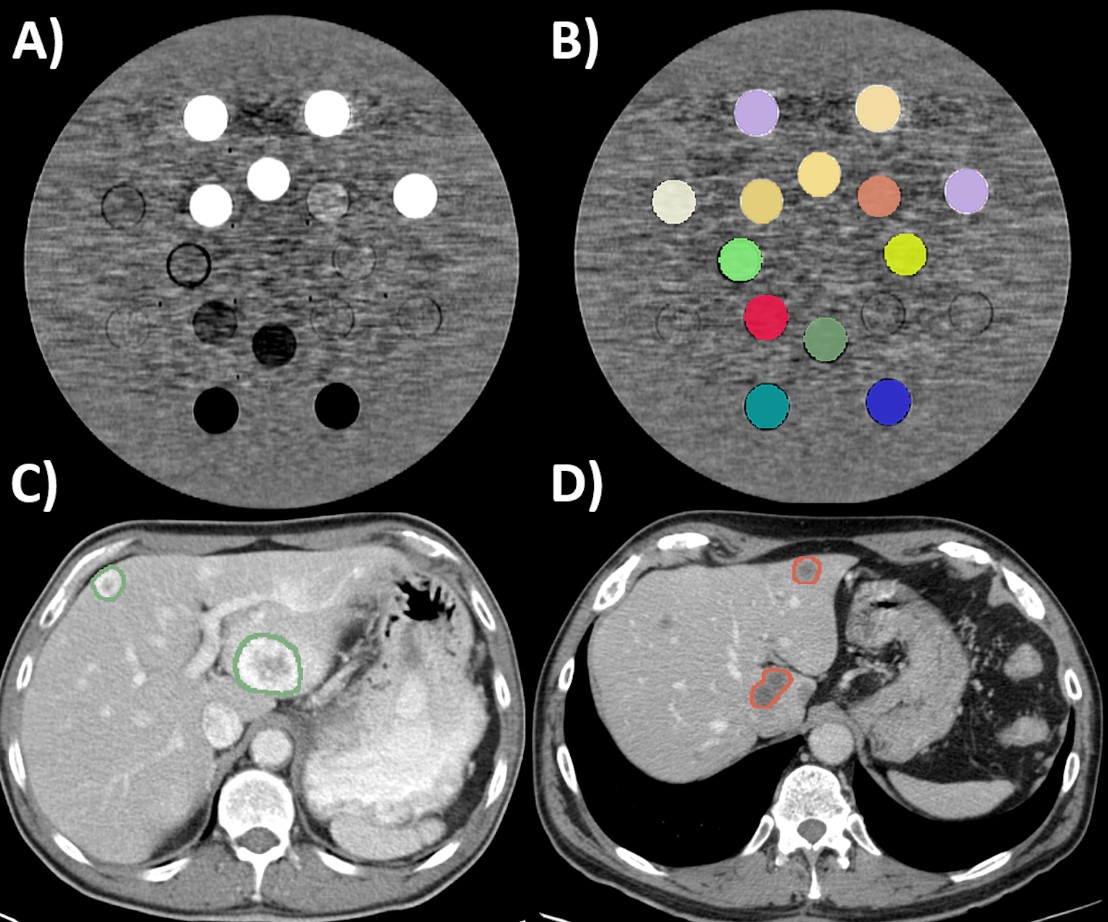
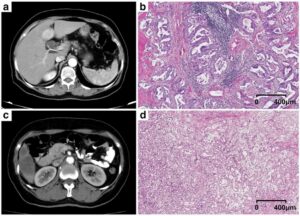
- ComBat batch effect correction reduced the CT-radiomics variability secondary to the slice thickness and convolution kernel, improving the capacity of CT-radiomics to differentiate tissues (in the phantom application) and the primary tumor type from liver metastases (in the clinical application).
Authors: Marta Ligero, Olivia Jordi-Ollero, Kinga Bernatowicz, Alonso Garcia-Ruiz, Eric Delgado-Muñoz, David Leiva, Richard Mast, Cristina Suarez, Roser Sala-Llonch, Nahum Calvo, Manuel Escobar, Arturo Navarro-Martin, Guillermo Villacampa, Rodrigo Dienstmann & Raquel Perez-Lopez