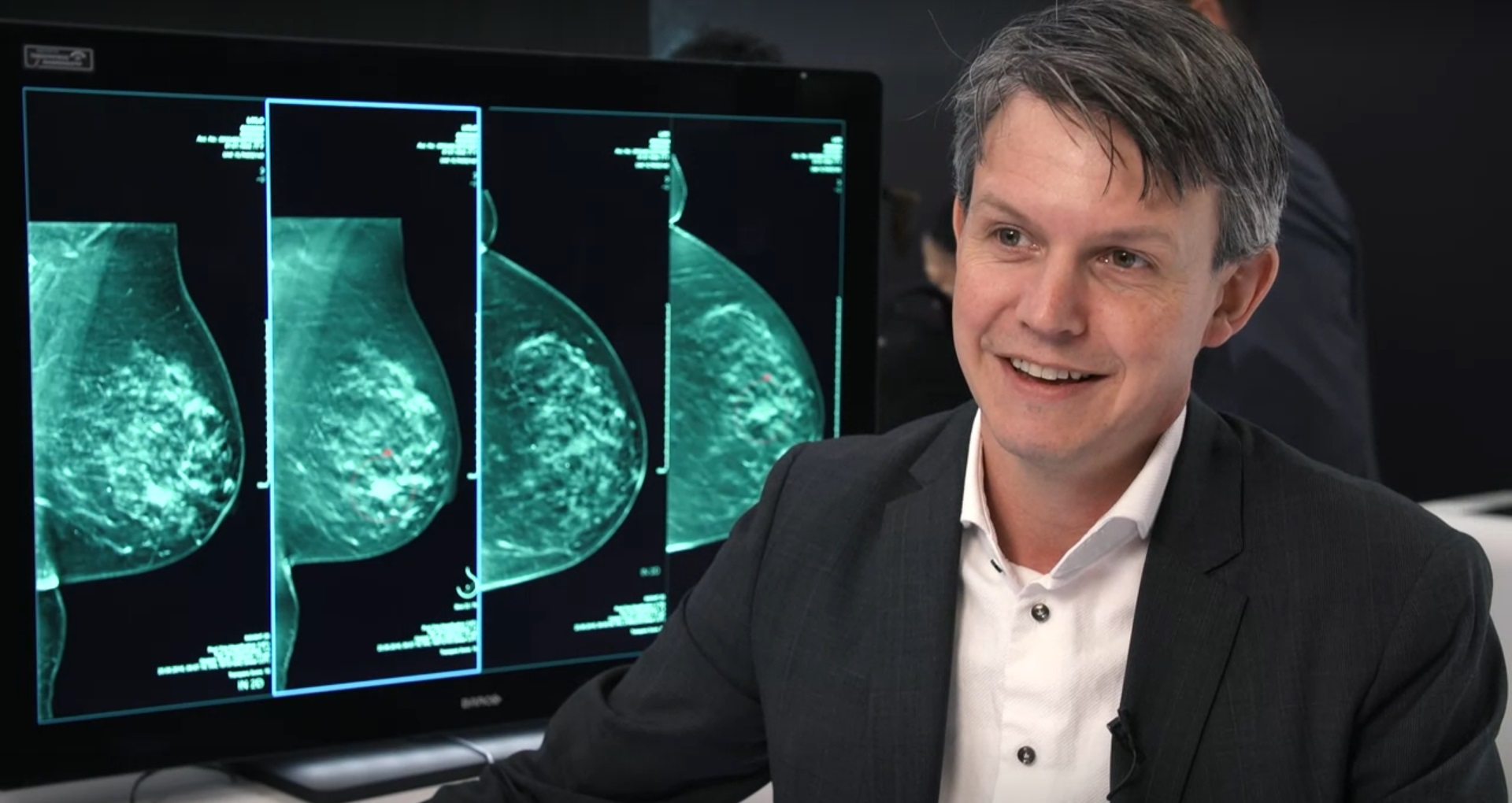
Artificial intelligence tools and technology are becoming more ingrained in the radiological discipline, but only recently in breast imaging, more specifically mammography and breast screening. Dr. Ritse Mann discussed with us the impact that AI is having on breast screening techniques and workflow, the current role it plays in hospitals and as an assistant to radiologists, and its future in breast screening.
What types of AI tools are available in relation to risk-based breast screening?
While further developed than in many other radiological subdisciplines, real AI applications for breast imaging, in general, are still relatively scarce. Only for mammography, and in recent years also for digital breast tomosynthesis (DBT) commercial, AI products have become available that are as good as expert breast radiologists in the detection of potentially cancerous lesions. These tools can be used as an aid to radiologists in the interpretation of mammography and DBT examinations and have been shown to increase accuracy, which is mainly important in the grey area of potential lesions that lead to doubt.
While it can be foreseen that these applications will also get a more decisive role in screening, for example by preselection of normal mammograms, or by acting as an independent second reader, currently this step has not yet been taken. It would mean that a substantial amount of images is seen by only 1 breast radiologist or by none at all, which brings ethical and juridical hurdles that are beyond the scientific discussion of the possibility. Likewise, these tools could be used to select a subset of cases that are more likely to harbour undetected cancers for supplemental or replacement screening techniques. In particular, additional ultrasound or MRI might be used in women with an increased risk based upon the case-based output of AI systems for mammography and DBT.
As a follow-up, how accessible is this technology for smaller or more rural clinics?
While it might seem that the use of these techniques is restricted to research centers and clinics with substantial assets, in fact, these techniques might lead to a cost-reduction. The simple use of concurrent AI for DBT might speed up the evaluation of the examination, therefore increasing the amount of cases that can be read by a single radiologist. It is also simply true that in a relatively small center it might be harder to ask a colleague for a second look. The simple fact that these systems are at the level of a breast radiologist provides such a second look without the need to actually have two experienced breast radiologists present.
For places where screening is currently not installed due to shortage of personnel, or where the backlog of work is simply increasing, the implementation of these systems may actually be the only way to enable a good screening practice, as training enough radiologists to do the job may simply be impossible due to a shortage of doctors and money.
How has AI made an impact on risk-based breast screening?
Currently very little. The automated density programs currently employed largely have a physical background and can therefore not be seen as true AI systems. However, especially in the US, selection of women for supplemental screening based upon automated density assessment has taken a big flight.
True AI applications are currently mainly marketed for concurrent reads and as such do not impact risk-based screening, albeit they may increase the overall quality of the screening program slightly. I am not aware of any large-scale dedicated screening program that has already incorporated AI for mammography or DBT as a truly independent or even stand-alone reader; albeit I am quite convinced that that will be the future for such a standardized task as screening.
How has AI changed the day-to-day clinical workflow?
Once again, currently only a little. AI applications currently available for breast imaging are aimed to aid in image interpretation and are generally most helpful for less experienced radiologists (particularly the available techniques for US and MRI that are in general not yet on par with the performance of expert radiologists). As such, they help in training and may prevent some overlook errors and misinterpretations. However, all cases undergo human evaluation too and therefore the effect is only minor.
Which types of tasks in risk-based breast screening can AI perform independently? Which types of tasks require a human component?
As even the most advanced AI applications for mammography and DBT have not been clinically validated on large scale consecutive screening cohorts, we are still a bit unsure about their performance as a real stand-alone reader. Consequently, the role of AI as an independent reader is really still limited. An important role here is for the large screening organizations that should make their data available for such tests. However, as it requires access to multiple data sources to get an adequate outcome and follow-up information, getting the data right is a cumbersome and time-intensive process that is not in the core objectives of these organisations. This comes on top of the above mentioned ethical and juridical difficulties and currently forms a major barrier for the actual implementation of AI techniques that have the ability to actually improve the quality of care and simultaneously reduce the costs.
In the clinic, breast imaging has an important emotional component. Women are in general concerned that they might have breast cancer, and many are well aware of the hardships that come with treatment and the realistic risk of dying from the disease. Hence, explanation of findings and eventual required further steps requires acknowledgement of these feelings and empathy, which is something I do not believe can easily be taken over by computers.
Are there any particular breast screening-related tasks where AI outperforms the radiologist?
As the performance of the best systems is at the level of breast radiologists or slightly above, the overall gain in performance of a screening program as such will only be minor. However, the huge advantage of using a computer to evaluate the images rather than the human is that the speed can be much higher, which means that in principle, screened women, even in population-based screening programs, could get the report of the examination the minute they exit the dressing room.
Obviously, if we change to other screening methods (for example MRI), it is required that AI techniques are developed along with the technical advances of the new technique to assure that these systems remain available.
How can AI help to better allocate resources for breast screening?
I think I actually already discussed that above. There is currently a massive human effort in screening that could be largely reduced by having a computer-based assessment. This will massively change the role of the breast radiologist as his/her role in screening will diminish. However, the clinical part of the job will likely not be affected much by the use of AI (and due to the detection of ever-smaller cancers, it might even increase), so I am not really concerned that the adoption of AI will lead to the disappearance of our profession (albeit we might need fewer people).
How does AI technology approach risk-based breast screening compared to other strategies (e.g. population-based screening)?
Currently-available AI tools are clearly developed for population-based screening. They might be used for risk-based screening, as especially the case-based scores entail a specific risk that a cancer might be present that may outperform, for example, simple density evaluation, but it is a bit out of the scope of their original intent. The combination of image-based evaluation with personal risk factors of the patient that might be obtained through questionnaires, or even from blood samples, may likely further improve the differentiation of risk categories. Still, even the best risk-models only achieve an area under the curve of around 0.7, which means that it currently remains very hard to select women that do not benefit from screening at all, or subgroups of women that do not benefit from the use of better screening techniques than simple mammography. In the end, these choices are still mainly politically driven and made based upon cost-effectiveness models. I strongly believe that women should themselves also get a voice in how they are being screened, after an explanation of the benefits and disadvantages of the different techniques. Actually, AI tools might make it possible to provide them with better insight than is achievable with current methods.
What does the future of breast screening look like when considering AI’s current role in radiology?
I strongly believe that AI will become an integral part of the radiological evaluation techniques. In other words, AI for mammography or DBT will simply be part of the mammography machine, and the output of an examination will likely be not only an image, but an image with an AI-based report.
For the development of other screening techniques, this will mean that it is of utmost importance to develop the AI algorithms alongside the development of the modalities. It should be noted that current AI tools for mammography that perform well are developed using thousands of cases. These will not be available for new techniques; hence, training should be feasible using much fewer examples as can also be done by humans. This will be a major hurdle that needs to be overcome by the AI community, otherwise, AI might become a limiting factor of innovation rather than a driver.
Learn more about the importance of breast screening on the Siemens Healthineers mammography website.
 Ritse Mann MD, PhD, obtained his medical degree at the University of Utrecht in the Netherlands in 2004. In 2005 he started a PhD project to the value of breast MRI in invasive lobular carcinoma at the Radboud University Medical Centre in Nijmegen. From 2008 to 2013 he trained as a resident in radiology, followed by a fellowship in interventional radiology at the same institution, where he now works as a breast and interventional radiologist. He is, since 2010, responsible for the clinical breast research at the radiology department of the Radboudumc. The breast imaging research group of the Radboudumc is one of the largest dedicated groups in Europe with large preclinical arms in x-ray development, ultrasound and artificial intelligence. Consequently, Dr. Mann’s research has a specific focus on the evaluation and implementation of novel breast imaging techniques. He also has a strong personal interest in breast MRI with a focus on the implementation of new sequences for breast screening.
Ritse Mann MD, PhD, obtained his medical degree at the University of Utrecht in the Netherlands in 2004. In 2005 he started a PhD project to the value of breast MRI in invasive lobular carcinoma at the Radboud University Medical Centre in Nijmegen. From 2008 to 2013 he trained as a resident in radiology, followed by a fellowship in interventional radiology at the same institution, where he now works as a breast and interventional radiologist. He is, since 2010, responsible for the clinical breast research at the radiology department of the Radboudumc. The breast imaging research group of the Radboudumc is one of the largest dedicated groups in Europe with large preclinical arms in x-ray development, ultrasound and artificial intelligence. Consequently, Dr. Mann’s research has a specific focus on the evaluation and implementation of novel breast imaging techniques. He also has a strong personal interest in breast MRI with a focus on the implementation of new sequences for breast screening.
Ritse Mann is an executive board member of the European Society of Breast Imaging (EUSOBI) since 2015, co-chairperson of the young club committee and member of the scientific committee, leading the annual scientific abstract evaluation. He also serves as lead section editor for the women’s imaging/breast imaging section of the European Journal of Radiology (EJR) and is member of the editorial board of the ESR’s Education on Demand program. He was recently appointed as a member of the scientific advisory board of the Dutch cancer society (KWF) and is a member of the EuroAIM working group on evidence-based radiology.